Dr. Götz Schuck
A Crystallographer’s Website
Rubrene
Rubrene (5,6,11,12-tetraphenylnaphthacene) is a red colored polycyclic aromatic hydrocarbon. It has the appearance of a red crystalline powder. Rubrene is used as a sensitiser in chemoluminescence. In lightsticks it is used to produce yellow light. It is an organic semiconductor, used in OLEDs and OLED-based displays. Single-crystal transistors can be prepared using crystalline rubrene. Crystals of rubrene and other organic semiconductors are generally grown in a modified zone furnace on a temperature gradient, by a technique known as Physical Vapor Transport. This method was introduced in 1997 by R. A. Laudise, Christian Kloc et al.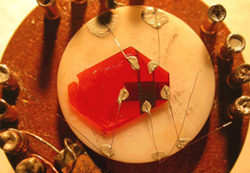
Rubrene holds the distinction of being the highest mobility organic semiconductor, with Podzorov et al. recording room-temperature field-effect mobilities of ~30cm2 / Vs using a novel air-gap dielectric architecture. It has also been employed to demonstrate the Hall Effect in rubrene, cited (along with photoconductivity experiments) as evidence of diffusive, band-type transport in organic crystals.
Reference:
Picture: Dr. Vitaly Podzorov; Text: Wikipedia
Crystallography
The crystal structures of two rubrene derivatives (5,11-BTBR (A) and 5,12-BTBR) were elucidated in order to understand the changes of the electric transport properties of the two 5,11-BTBR polymorphs (A and B). This work was a cooperation between the Physics of New Materials Group (ETH Zurich), Ciba Specialty Chemistry (Switzerland) and the High Pressure Materials Synthesis Group (ETH Zurich).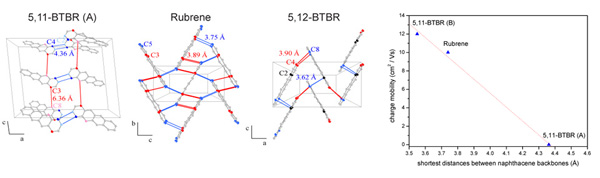
Reference:
Poster:
Crystal structures of two rubrene derivatives
Authors: Götz Schuck, Simon Haas, Ulrich Berens and Hans-Jörg Kirner
Conference: ECM-24: Marrakech, Morocco: 22-27 August 2007 , PDF
Publication:
Authors: Götz Schuck, Simon Haas, Ulrich Berens and Hans-Jörg Kirner
Conference: ECM-24: Marrakech, Morocco: 22-27 August 2007 , PDF
High charge-carrier mobility and low trap density in a rubrene derivative
Authors: S. Haas, A. F. Stassen, G. Schuck, K. P. Pernstich, D. J. Gundlach, B. Batlogg, U. Berens, H.-J. Kirner
Journal-ref: Phys. Rev. B 76 , 115203 (2007) , PDF
Publication:
Authors: S. Haas, A. F. Stassen, G. Schuck, K. P. Pernstich, D. J. Gundlach, B. Batlogg, U. Berens, H.-J. Kirner
Journal-ref: Phys. Rev. B 76 , 115203 (2007) , PDF
5,11-Bis(4-tert-butylphenyl)-6,12-diphenylnaphthacene (form A)
Authors: Götz Schuck, Simon Haas, Arno F. Stassen, Ulrich Berens and Bertram Batlogg
Journal-ref: Acta Cryst. (2007). E63 , o2894 , PDF
Publication:
Authors: Götz Schuck, Simon Haas, Arno F. Stassen, Ulrich Berens and Bertram Batlogg
Journal-ref: Acta Cryst. (2007). E63 , o2894 , PDF
5,12-Bis(4-tert-butylphenyl)-6,11-diphenylnaphthacene
Authors: Götz Schuck, Simon Haas, Arno F. Stassen, Hans-Jörg Kirner and Bertram Batlogg
Journal-ref: Acta Cryst. (2007). E63 , o2893 , PDF
Authors: Götz Schuck, Simon Haas, Arno F. Stassen, Hans-Jörg Kirner and Bertram Batlogg
Journal-ref: Acta Cryst. (2007). E63 , o2893 , PDF